At the Diabetes Center of Metropolitan Hospital, a specialized Type 1 Diabetes (insulin-dependent) clinic is in operation. In this clinic, advanced technologies for continuous glucose monitoring are already in use, including the blinded Envision Pro system (Medtronic), the flash glucose monitoring system (FreeStyle Libre), and real-time continuous glucose monitoring (CGM) systems such as the Guardian Sensor 3, which can be used in combination with the MiniMed 640G insulin pump currently available in Greece. Data from these systems can be appropriately stored and accessed: Envision Pro data through a dedicated application, FreeStyle Libre data via the scanner and then transferred to the physician’s computer using a USB port, and Medtronic CareLink data online in a personalized cloud-based file, allowing remote transmission to the treating physician. This enables the physician to evaluate the data, communicate with the patient, and adjust the treatment regimen with the aim of optimizing glycemic control, reducing glycemic variability, and ultimately preventing complications of Type 1 Diabetes.
Type 1 Diabetes: Symptoms and standard treatment
Type 1 Diabetes Mellitus (also known as juvenile diabetes or insulin-dependent diabetes, as it was previously called) typically appears suddenly, often with striking symptoms, and in the majority of cases, at a very young age. It accounts for approximately 5–10% of all cases of diabetes mellitus.
The condition is caused by an autoimmune destruction of a specific group of pancreatic cells, called β-cells, which are responsible for producing insulin.
In infancy and early childhood, the destruction of β-cells tends to be rapid, frequently leading to diabetic ketoacidosis (DKA), which is often the first manifestation of the disease. Diabetic Ketoacidosis (DKA) is a serious condition in which the patient presents with polyuria, polydipsia, weight loss, nausea, vomiting, abdominal pain, dehydration, and altered levels of consciousness. Additional signs may include dry mouth and mucous membranes, tachycardia, hypotension, and deep, frequent respirations that are characteristically malodorous (resembling the smell of rotten apples). Depending on how quickly the condition is recognized, the child may be fully alert, confused, or even in a coma.
The progression of DKA is rapid, with symptoms developing in less than 24 hours. In older individuals, the disease tends to evolve more slowly due to a less aggressive rate of β-cell destruction. To date, the only established treatment for Type 1 Diabetes, apart from experimental pancreatic islet transplantation, is insulin therapy.
More specifically, management relies on intensive insulin regimens combining: Basal insulins (long-acting insulins such as Lantus, Toujeo, Abasaglar, and Tresiba), which cover the patient’s basic insulin needs and Prandial insulins (rapid-acting insulins such as NovoRapid, Humalog, Apidra, and the ultra-rapid Fiasp, as well as older insulins like Actrapid and Humulin Regular), which are used to cover glucose and starch intake from meals or to quickly correct episodes of hyperglycemia.
Over the past decade, there has been a sharp rise in the use of insulin pumps, both in the United States and in Europe. Globally, between 20–25% of patients with Type 1 Diabetes use insulin pumps in the U.S., with adoption rates reaching up to 60% in specialized pediatric centers in Germany. Wider use of insulin pumps has been facilitated by insurance coverage for the devices and their consumables in several countries.
Innovative Treatment for Type 1 Diabetes – Insulin Pumps
What is an insulin pump?
An insulin pump is a device smaller than a typical mobile phone that delivers insulin continuously under the skin. It consists of several key components:

- Electronic Unit: This contains the pump’s programming and sends commands to the reservoir plunger to deliver insulin.
- Reservoir (Tank): The storage container for insulin.
- Plastic Tubing: Connects the insulin pump to the cannula, through which insulin is administered into the patient’s body.
- Cannula: Inserted subcutaneously (under the skin) with the help of an introducer needle, which is removed after insertion. The cannula remains in place in the fatty tissue where insulin injections are usually administered and is secured to the skin with adhesive.
- Monitor: Displays information on insulin delivery and allows management of continuous subcutaneous insulin infusion.
Some insulin pumps include a glucose sensor that communicates wirelessly with the pump (as in the Medtronic 640 pump).

In Greece, insulin pumps from the following manufacturers are available: Medtronic (Paradigm 640), Roche (Spirit Combo), and Dana.
Advantages of the Insulin Pump
- Greater freedom in daily routines.
- Corrective insulin doses for hyperglycemia can be administered at the press of a button, without the need for an additional injection.
- Similarly, pre-meal insulin doses can be delivered without a new injection.
- Ability to set different insulin dosing programs, allowing better management of complex meals.
- Pump suspension in case of hypoglycemia (a feature not available in all pumps). Computer-assisted dosing programs to facilitate user management.
- Capability to deliver micro-doses of insulin.
The use of an insulin pump can be especially beneficial for: Infants and newborns, adolescents with appetite disorders, athletes in competitive sports, individuals with needle phobia, workers with rotating or irregular shifts, people experiencing the “dawn phenomenon” (elevated blood glucose in the morning) and individuals with fluctuating daily physical activity, such as farmers.
Challenges in using the Insulin Pump
Some patients may struggle to accept wearing a foreign device on their body or may not wish to disclose their condition to others. Additionally, audible alerts from the pump can be bothersome for certain individuals. Finally, the insulin pump is an expensive device; however, it is fortunately covered for patients with type 1 diabetes (insulin-dependent) by health insurance in many countries, including Greece.
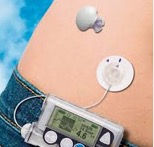
Sensor Augmented Pump (SAP)
In Greece, the Medtronic 640G insulin pump is available, which works in combination with the Guardian Sensor 3 continuous glucose monitor (CGM). The advantages and key strengths of this system are that users, by responding to the sensor’s alerts and readings, can correct their blood glucose with more frequent, smaller insulin doses made especially easy and painless using the insulin pump ultimately achieving lower HbA1c levels. Additional features include:
- Suspension of insulin delivery at a preset low glucose threshold to prevent or more easily correct hypoglycemia.
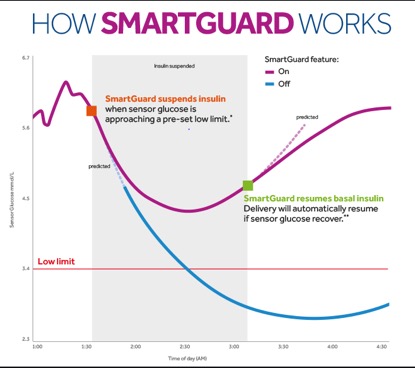
- Pre-low insulin suspension, with the additional capability of predictive suspension for anticipated low glucose (PLGM – Predictive Low Glucose Management, also known as Smart Guard software).
- Insulin delivery at high glucose levels, in addition to the pre-low suspension feature (available in the 670 pump and newer models connected with the 4th generation Guardian Sensor).
The future of monitoring patients using Insulin Pumps
In recent years, a few studies have suggested potential benefits of insulin pump use in managing Type 2 Diabetes (non-insulin-dependent) and even gestational diabetes, particularly when combined with a glucose sensor. However, the cost of using pumps in these cases is not typically covered by insurance, and further research is needed to draw definitive conclusions.
Another capability offered by sensor-equipped insulin pumps is remote patient monitoring. Patients can upload continuous glucose monitoring data and other relevant information to the cloud, allowing their physician to access it online and provide remote medical guidance, potentially including live teleconsultation. This is especially valuable for patients living in remote areas or on islands.
Continuous Glucose Monitoring Systems (CGMs)
CGMs are devices that allow continuous measurement and monitoring of glucose levels in the subcutaneous tissue, providing a more comprehensive view of glucose trends over time. These systems consist of the following components:
- Subcutaneous glucose sensor: A catheter inserted under the skin that continuously measures glucose levels.
- Transmitter: A coin-sized device attached to the sensor-catheter and secured to the skin with adhesive. It stores and/or wirelessly transmits the sensor data to another device via telemetry.
(Φωτογραφία φεύγει) - Monitoring device (monitor): Displays the glucose data for real-time tracking. Some CGM systems, known as “flash” or blind systems, do not include a monitor. When a CGM is paired with an insulin pump, the pump’s display serves as the receiver.
CGM Types
- Blind Systems
These are simple continuous glucose monitoring systems without a display, such as the iPro and Envision (Medtronic). They do not provide real-time glucose readings to the user but record and store the data. The user can continue daily activities without being affected by sensor values. The sensor has a lifespan of 7 days, after which it is removed and the data are transferred to a computer for analysis.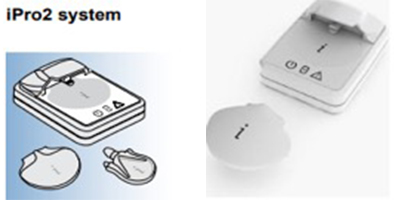
- Flash Glucose Monitoring (Flash Technology – FreeStyle Libre)
This system consists of a coin-sized sensor and a scanner roughly the size of a mobile phone.
It does not provide audible alerts and does not require calibration with capillary blood. Glucose values are not displayed continuously; they are only shown when the sensor is scanned with the reader. - Real-Time Continuous Glucose Monitoring Systems (RT-CGMs)
n these systems, the sensor measures glucose in real time, typically every 5 minutes. The readings are transmitted via telemetry to a remote device, such as an insulin pump. They feature audible alerts for high and low glucose levels. Some systems, like the MiniMed 640G (Medtronic), can automatically adjust or suspend insulin delivery based on sensor readings to help prevent hypoglycemic episodes.
Insulin Pump & CGM Software and cloud technology
- Open-Source Platforms and DIY Hybrid Systems
Nightscout is an open-source software that allows access to continuous glucose monitoring (CGM) data via smart devices (smartwatches, phones, tablets) for anyone with authorized access. It supports multiple sensors (Dexcom, FreeStyle Libre, Medtronic) as well as insulin pumps, enabling real-time and remote glucose monitoring. This is particularly useful for relatives of children with Type 1 Diabetes. Nightscout was the foundation of the online social movement “We Are Not Waiting,” which consists of open communities of users and developers collaborating to help people with Type 1 Diabetes use existing devices for better glycemic control. With proper guidance, users can convert commercially available devices into personalized algorithms (Do It Yourself, DIY) to create closed-loop systems. Users adjust meal-time insulin as well as doses for exercise or illness. This process is time-consuming and requires technical knowledge, and users are solely responsible for the safety of these systems for personal use, as building systems for others is prohibited. -
Official Company Software using cloud technology
Several commercial platforms, such as CareLink (Medtronic), store patient data in secure online accounts that can be accessed by the treating physician with patient consent. The Guardian Connect sensor connects via Bluetooth to a mobile phone and through it to the internet. In 2015, Dexcom released software similar to Nightscout compatible with Android and Apple devices, enabling real-time monitoring of the Dexcom G5 sensor and transmission of data to up to five phones simultaneously. The Cellnovo micro-pump is controlled via a touchscreen mobile device, with data transmitted online to a platform where the patient can grant access to their healthcare provider and/or family members.